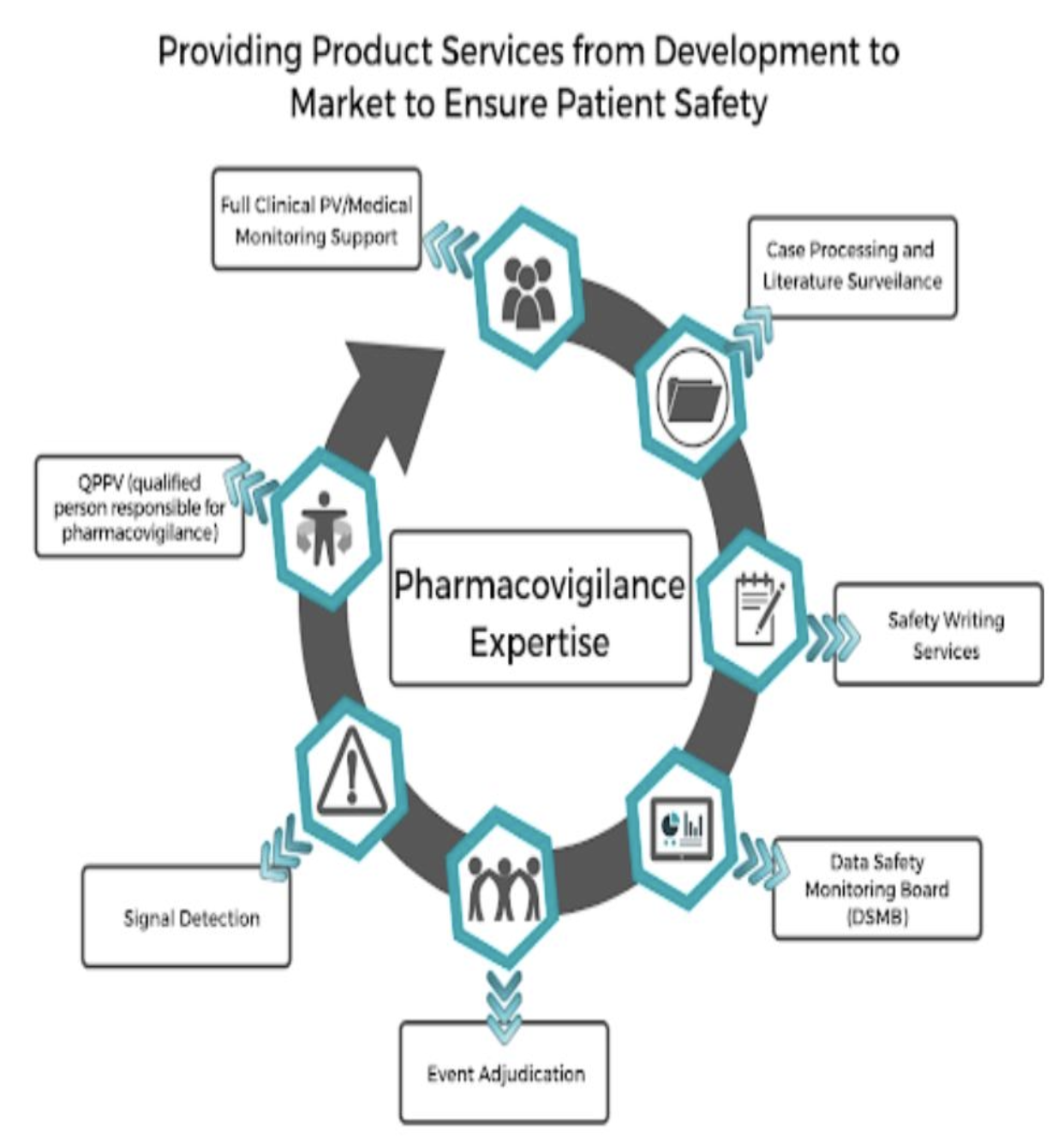
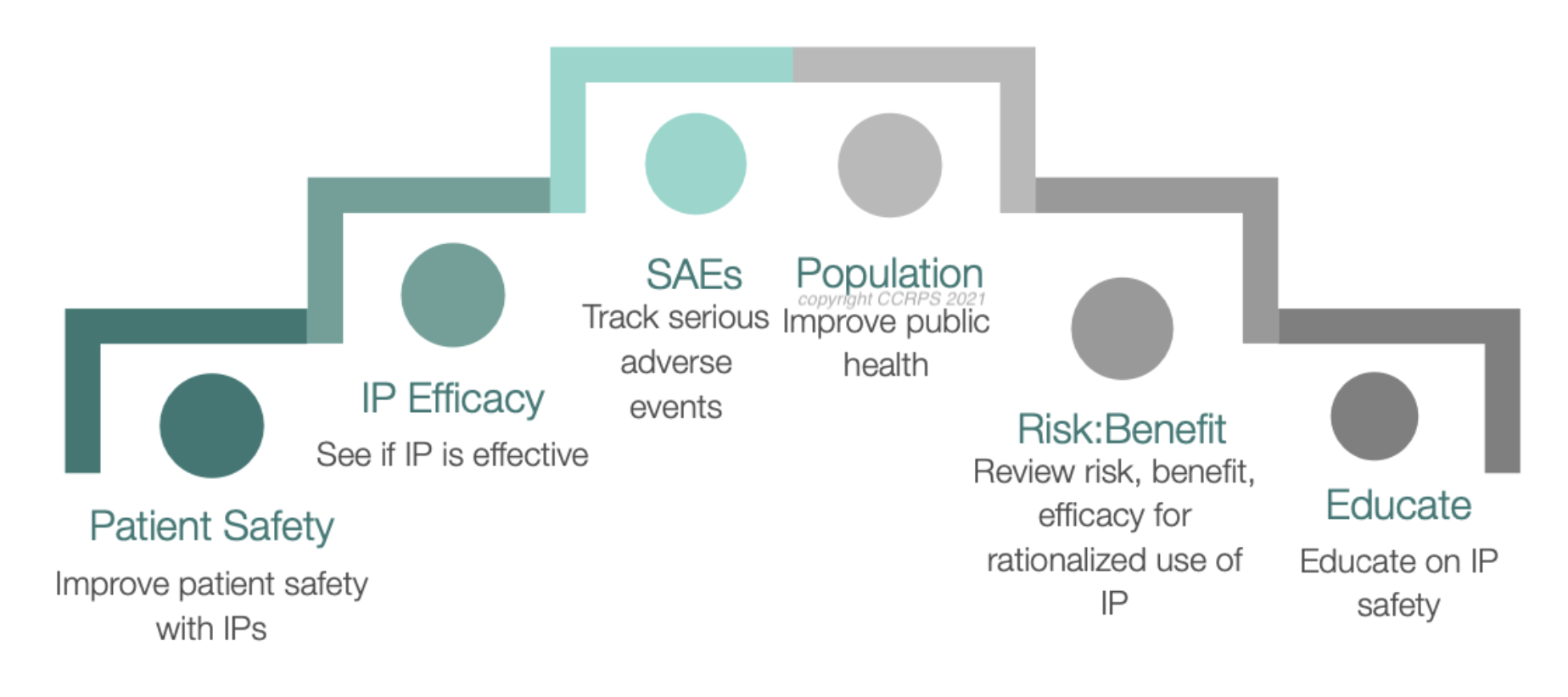
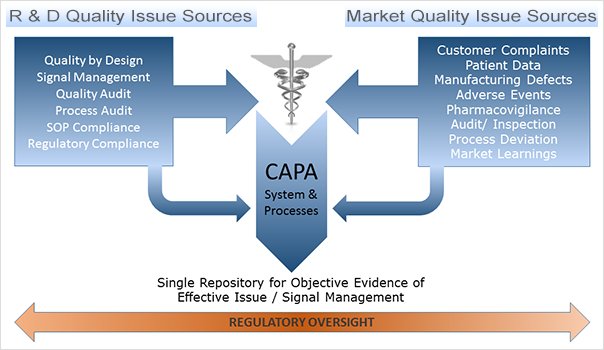
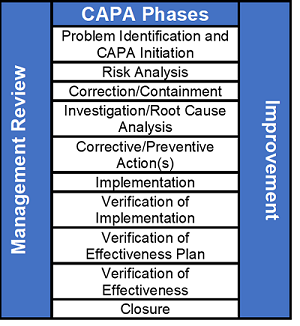
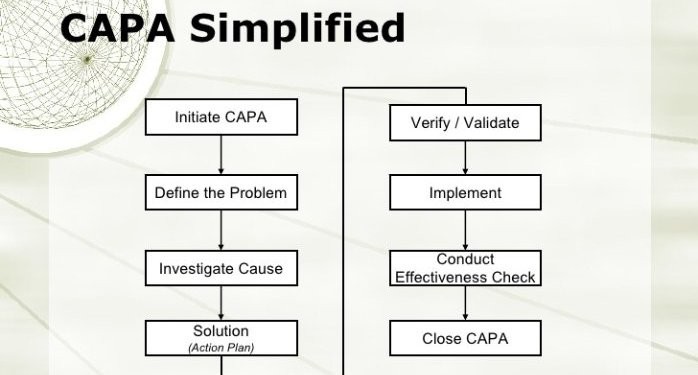
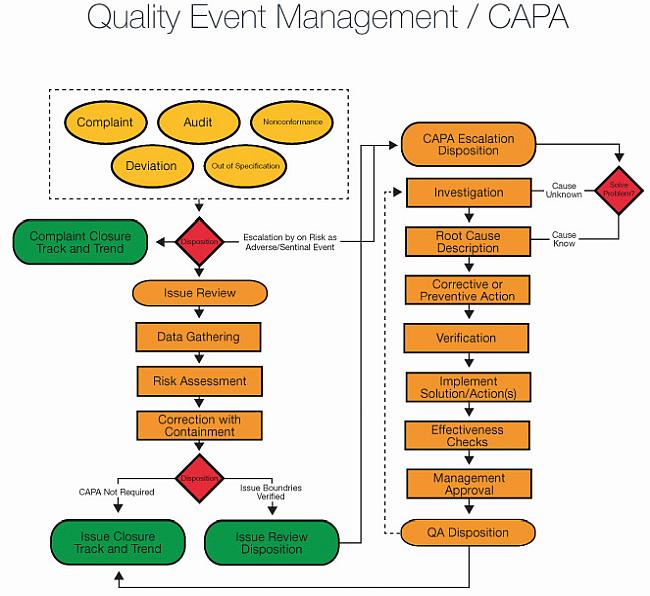
Root cause Analysis (RCA) & Corrective and Preventive action (CAPA) in MRCT dr. bhaswat chakraborty final

CAPA management. How to turn PV audit failure into success. - Pharmacovigilance, Auditing and Regulatory Services

Responding to Clinical Observations: Developing Robust Audit & Inspection CAPAs - Life Science Training Institute

Root cause Analysis (RCA) & Corrective and Preventive action (CAPA) in MRCT dr. bhaswat chakraborty final