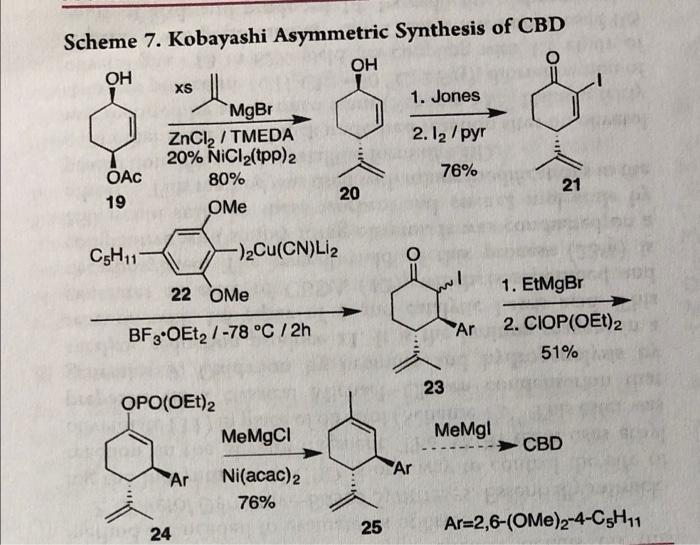
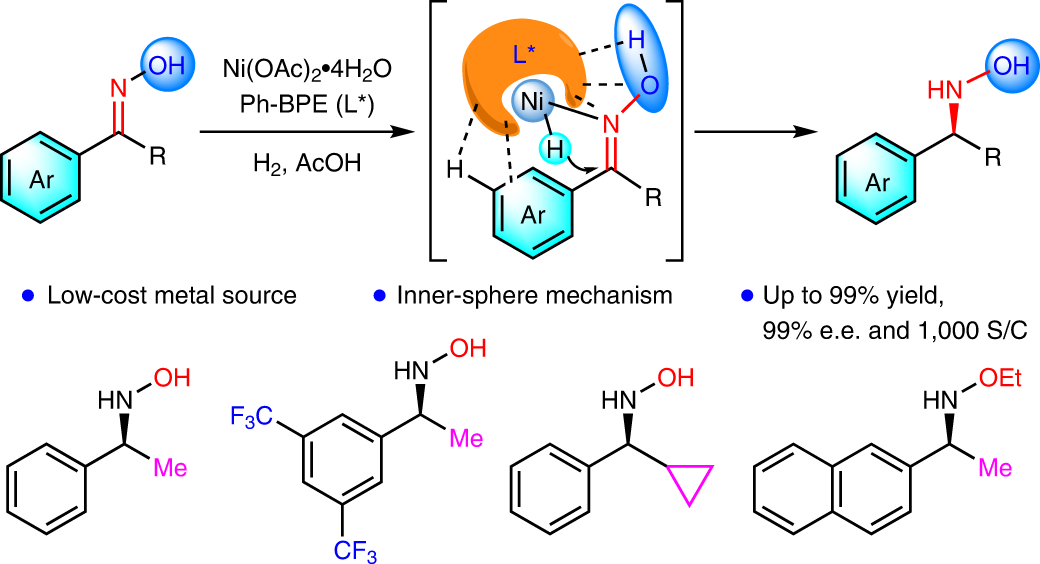
Diastereo- and Enantioselective Construction of Spirocycles by Nickel-Catalyzed Cascade Borrowing Hydrogen Cyclization | Journal of the American Chemical Society

Ligand-enabled Ni-catalyzed hydroarylation and hydroalkenylation of internal alkenes with organoborons | Nature Communications

Regulating the Synergistic Effect in Bimetallic Two‐Dimensional Polymer Oxygen Evolution Reaction Catalysts by Adjusting the Coupling Strength Between Metal Centers - Fa - Angewandte Chemie International Edition - Wiley Online Library

Emerging Nickel Catalysis in Heck Reactions: Recent Developments - Bhakta - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library

Dual electronic effects achieving a high-performance Ni(II) pincer catalyst for CO2 photoreduction in a noble-metal-free system | PNAS

Nickel-Catalyzed Heck Reaction of Cycloalkenes with Inert C—O Bonds of Aryl Carbonates and Aryl Sulfamates

Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling | Science

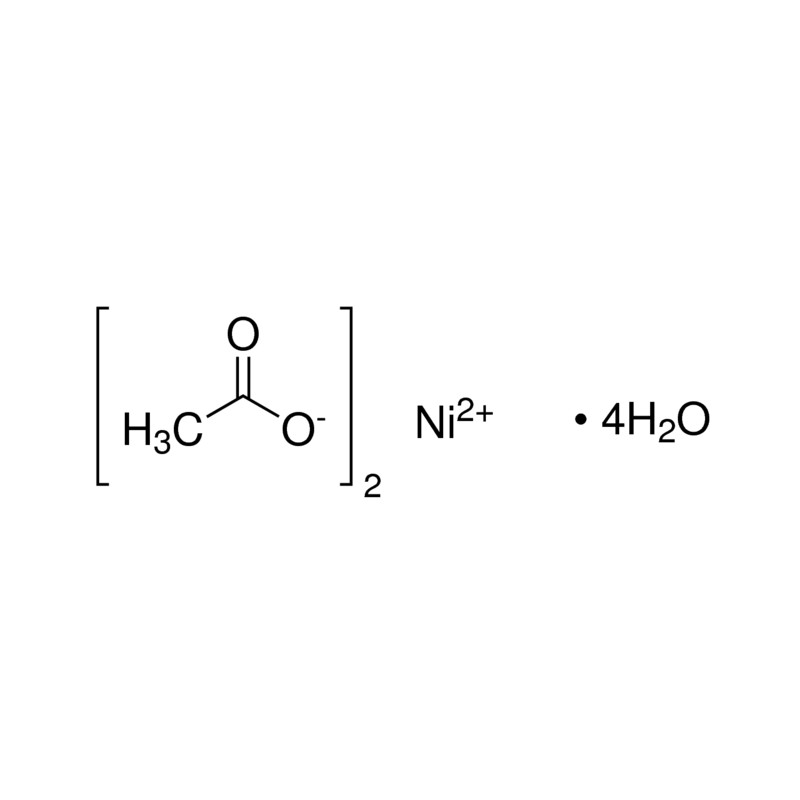
Ni(OAc)2: a highly efficient catalyst for the synthesis of enaminone and enamino ester derivatives under solvent‐free conditions - Liu - 2010 - Applied Organometallic Chemistry - Wiley Online Library
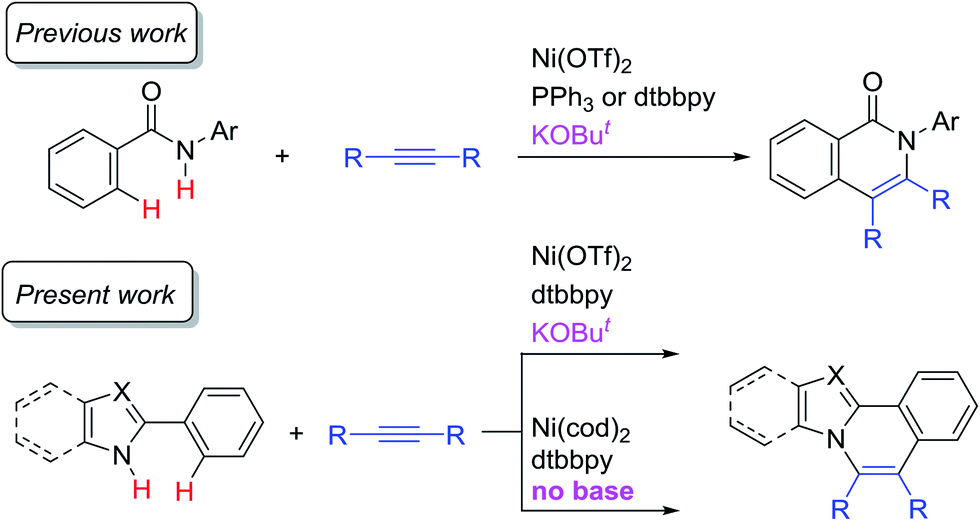
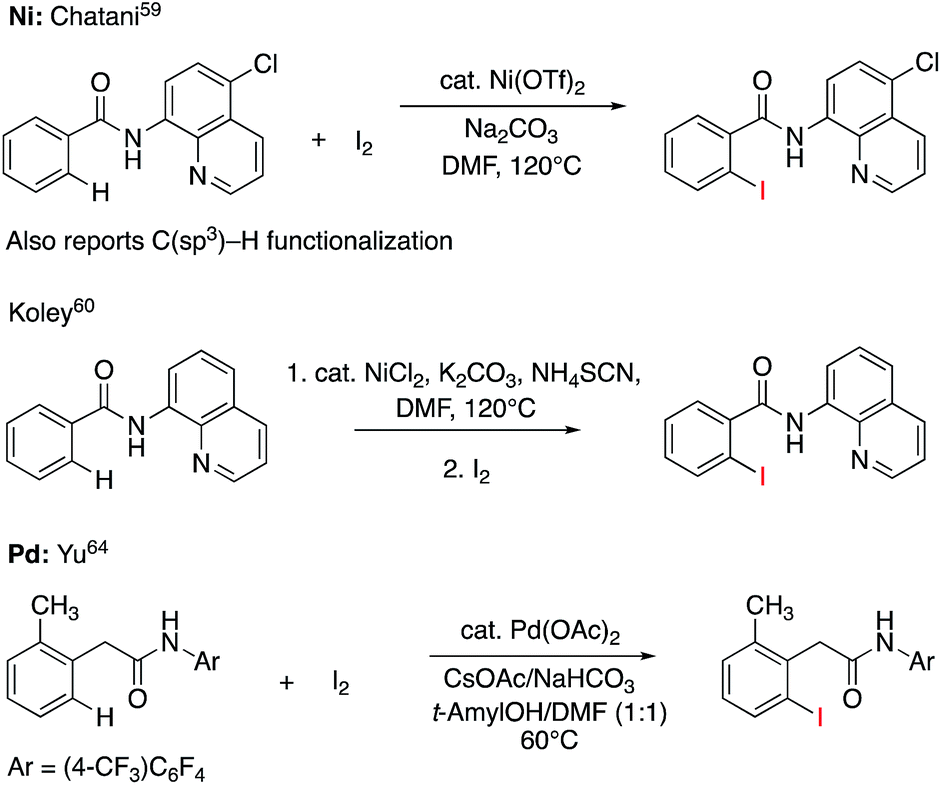
Nickel-catalyzed oxidative C–H/N–H annulation of N -heteroaromatic compounds with alkynes - Chemical Science (RSC Publishing) DOI:10.1039/C8SC05063E

Development of Nickel-Catalyzed Cross-Coupling of Alcohol Derivatives to Construct Carbon-Carbon Bonds
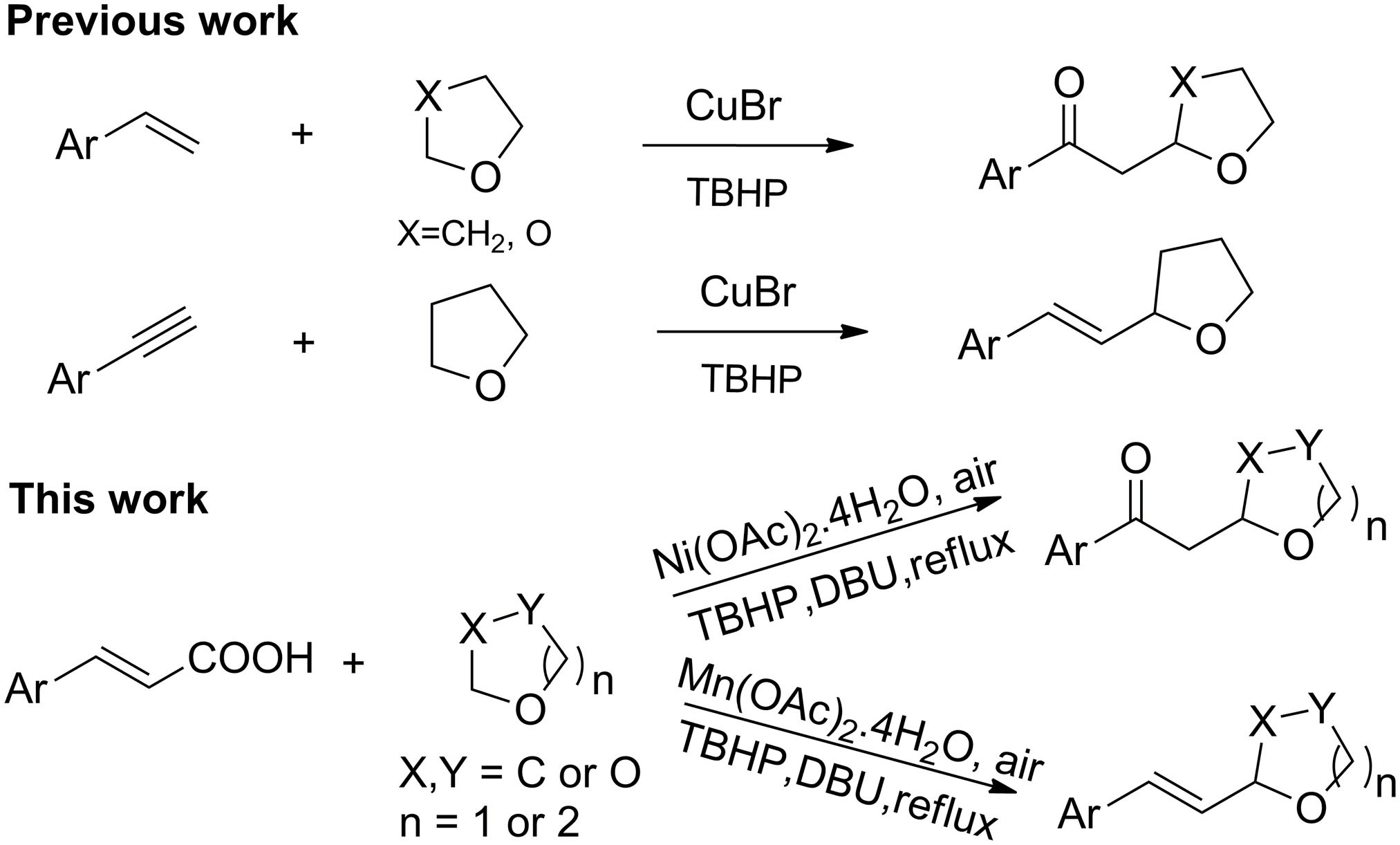
Selective Nickel- and Manganese-Catalyzed Decarboxylative Cross Coupling of Some α,β-Unsaturated Carboxylic Acids with Cyclic Ethers | Scientific Reports

The relationship between ligands and catalytic effect. The catalytic... | Download Scientific Diagram
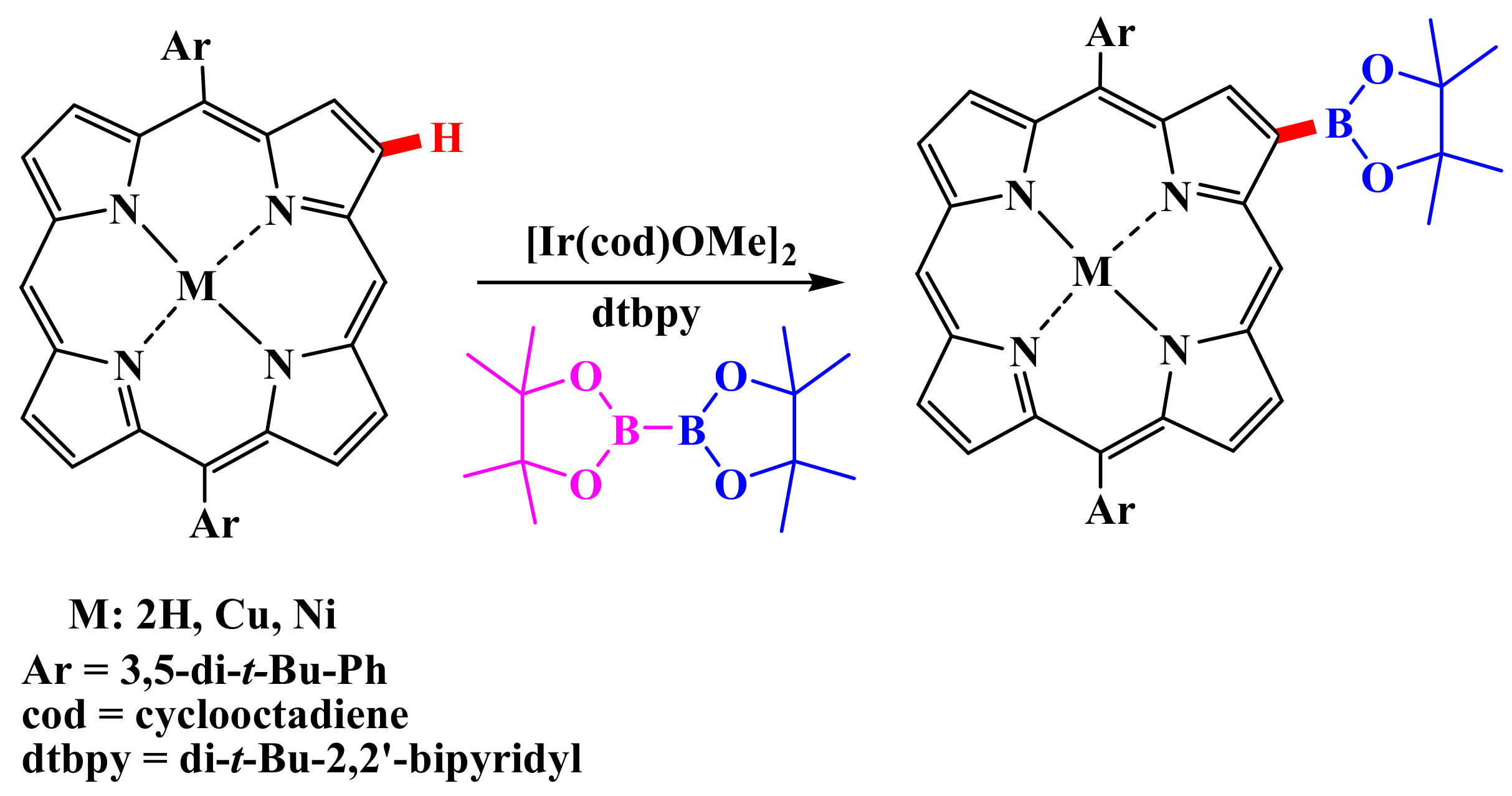
Inorganics | Free Full-Text | Functionalization of Porphyrins Using Metal-Catalyzed C–H Activation

Transition-Metal-Catalyzed Functionalization of Alkynes with Organoboron Reagents: New Trends, Mechanistic Insights, and Applications | ACS Catalysis
![2,6-Bis[(2R,4S,5S)-1-benzyl-4,5-diphenylimidazolidin-2-yl]pyridine 1223020-29-8 | TCI (Shanghai) Development Co., Ltd. 2,6-Bis[(2R,4S,5S)-1-benzyl-4,5-diphenylimidazolidin-2-yl]pyridine 1223020-29-8 | TCI (Shanghai) Development Co., Ltd.](https://www.tcichemicals.com/assets/cms-images/B3934ref.gif)
2,6-Bis[(2R,4S,5S)-1-benzyl-4,5-diphenylimidazolidin-2-yl]pyridine 1223020-29-8 | TCI (Shanghai) Development Co., Ltd.
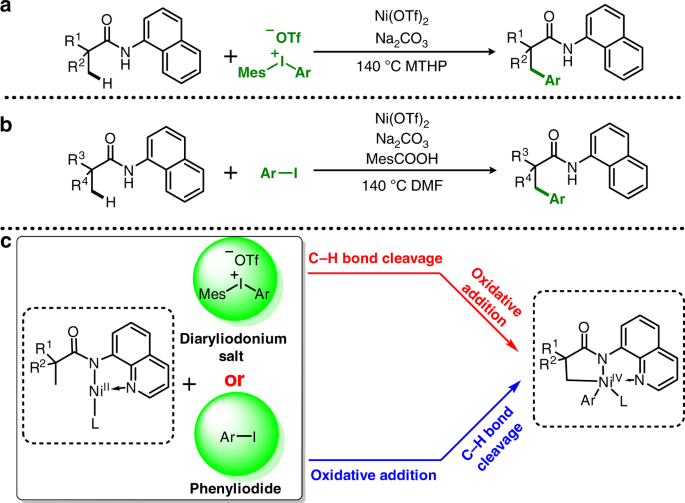
Theoretical study of FMO adjusted C-H cleavage and oxidative addition in nickel catalysed C-H arylation | Communications Chemistry