Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces

Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors | ACS Applied Materials & Interfaces

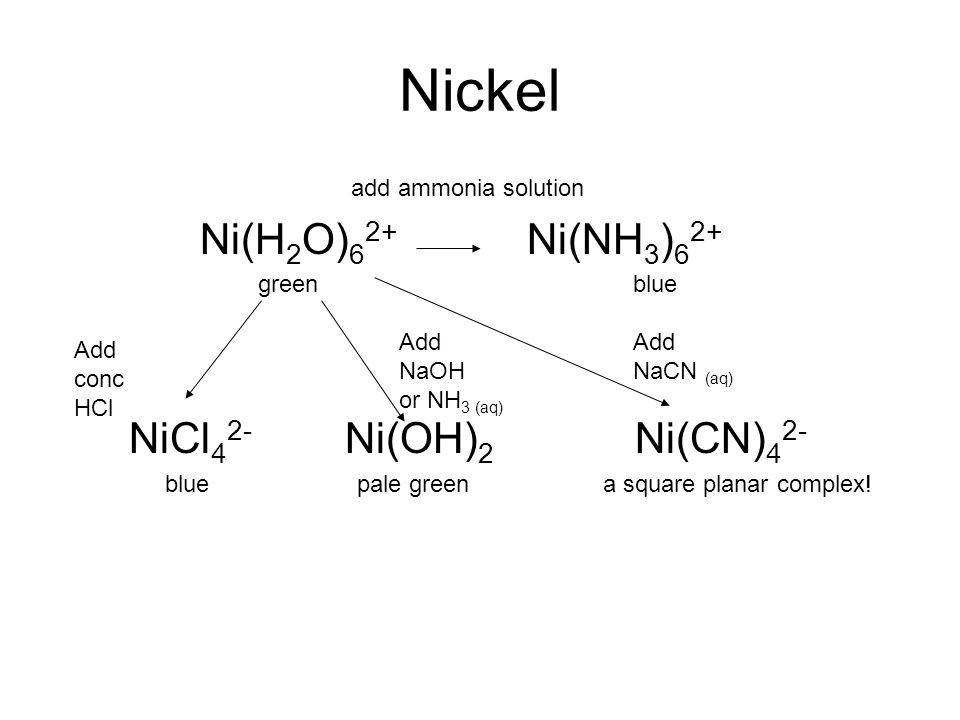
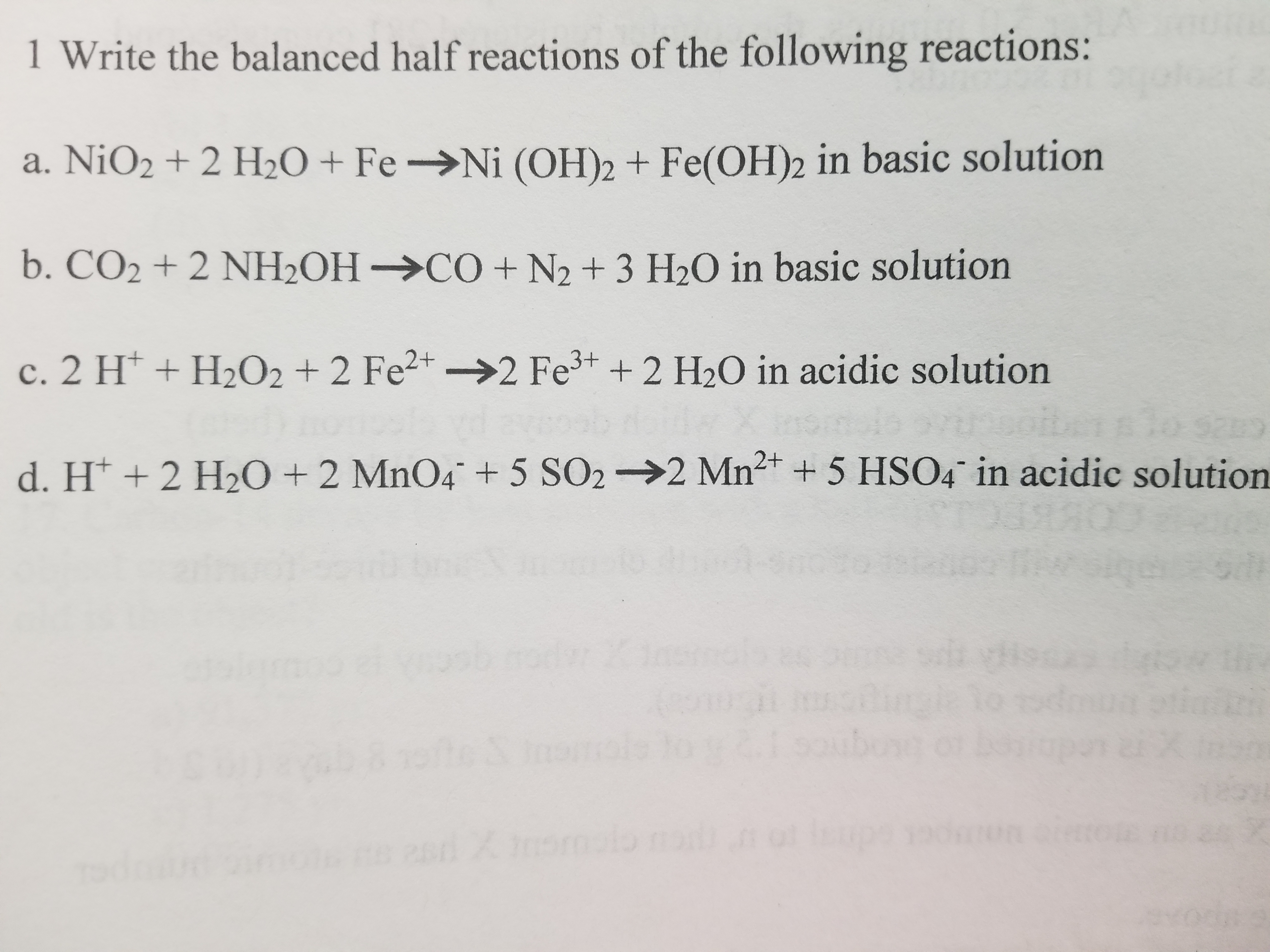
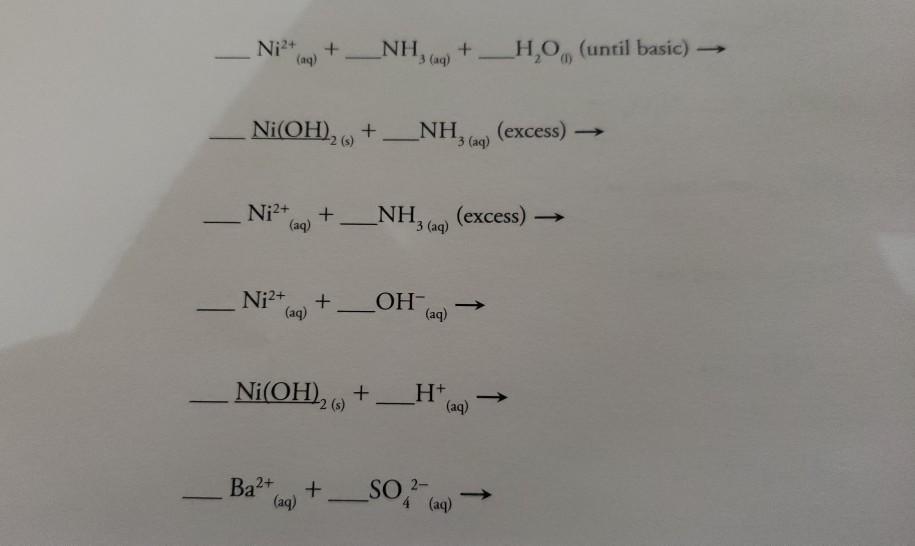
E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
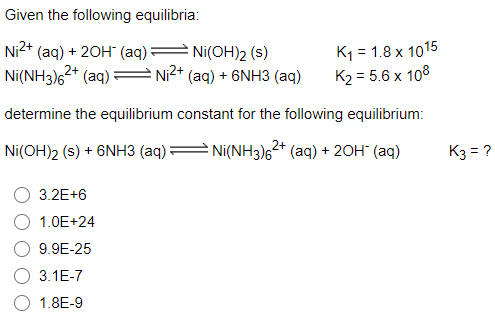
![SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light. SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [NiOHz)]2+ is green; while [Ni(NH3)]2+ is purple: Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6l2+ absorbs violet light [Ni(OHz)a]2+ absorbs red light.


2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)
![Ni(OH2)6]2+ Ni(OH2)6]2+](http://www.chemtube3d.com/images/gallery/inorganicsjpgs/Ni_OH2_62_.jpg)

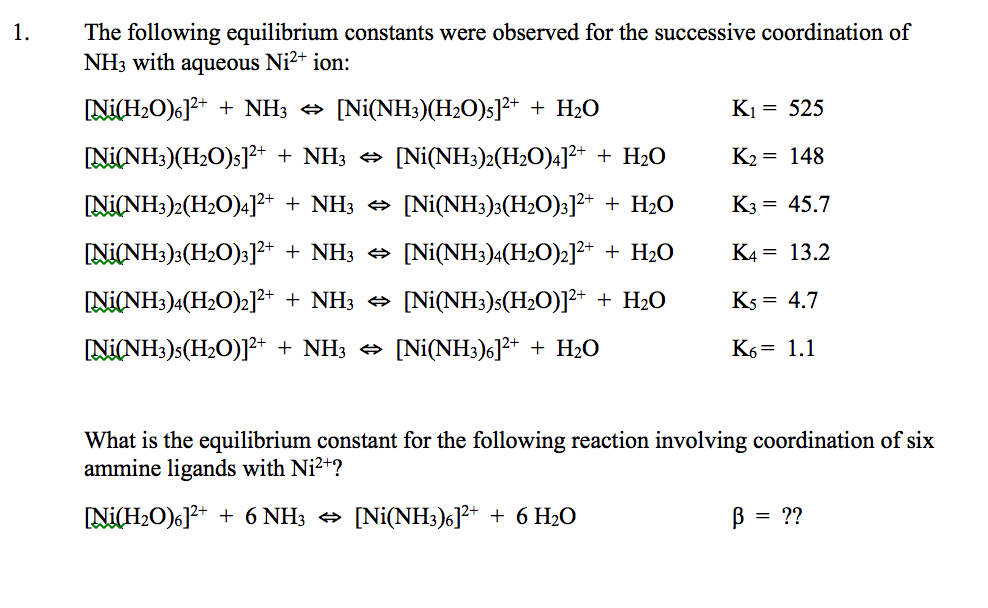
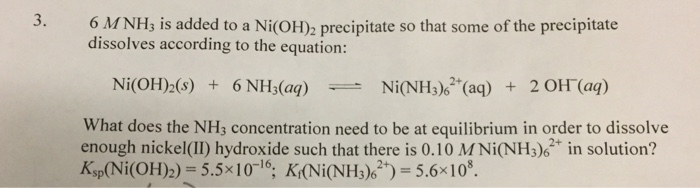
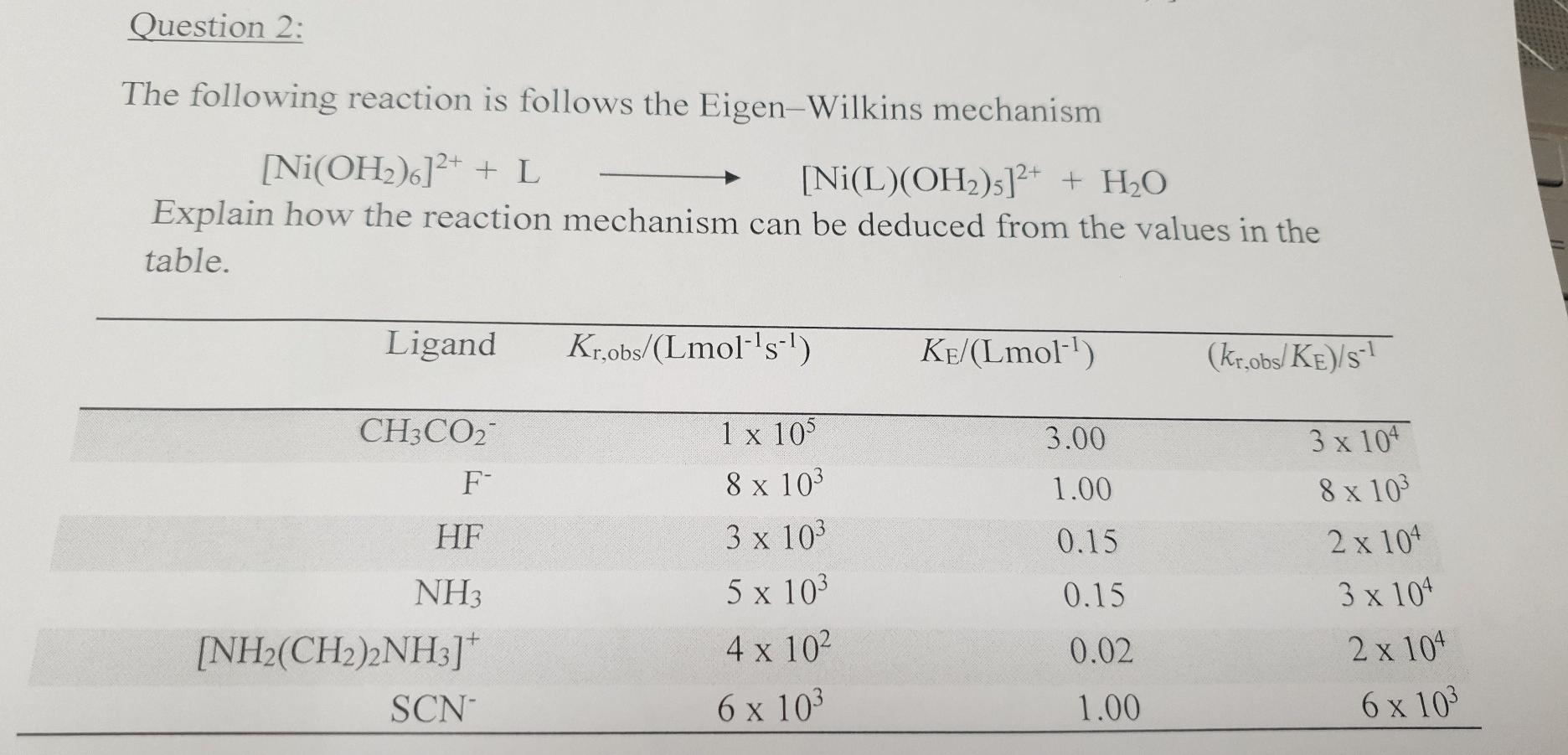
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)