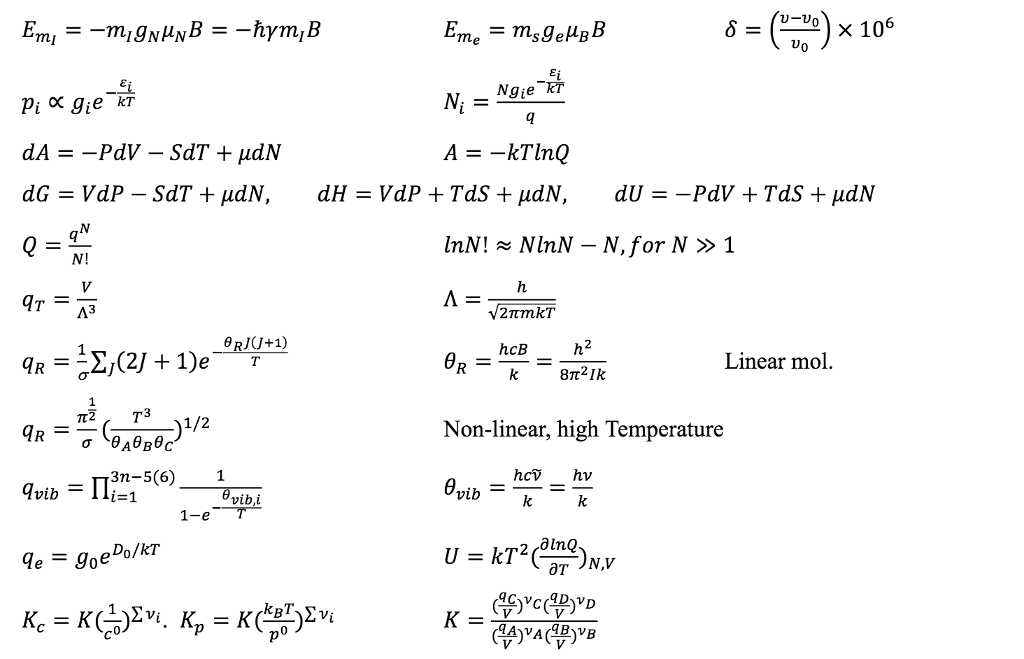
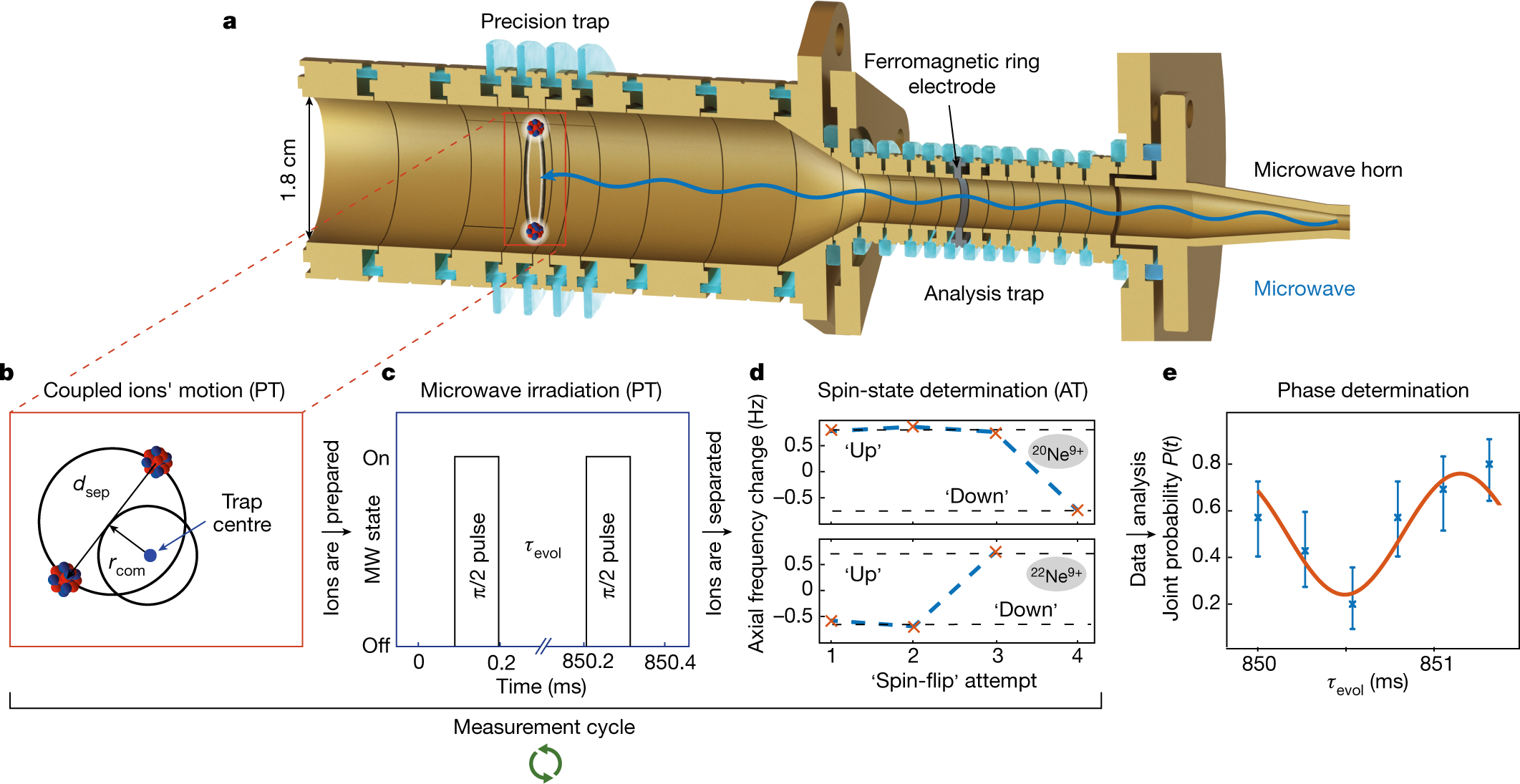
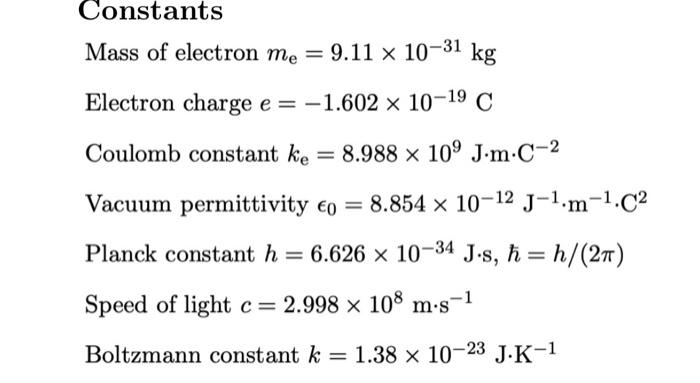
SOLVED: Data Sheet Speed of light =3X108 m -1 Planck's constant h = 6.626 X 10-34 J s Planck's constant, reduced h= h/2n 1.055 X 10-34 Js 6.582 x 10- 22 MeV

What will be the uncertainty in velocity of an electron when the uncertainty in its position is 1000 A ?
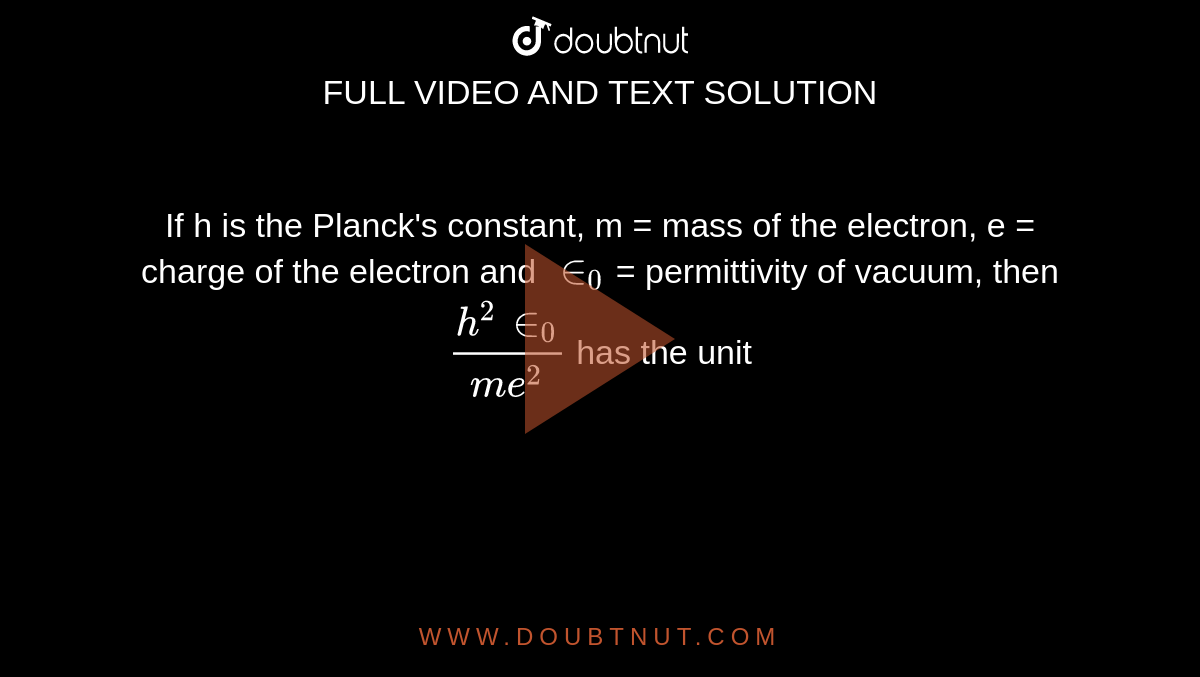
If h is the Planck's constant, m = mass of the electron, e = charge of the electron and in(0) = permittivity of vacuum, then (h^(2)in(0))/(me^(2)) has the unit

If h is the Planck's constant, m = mass of the electron, e = charge of the electron and in(0) = permittivity of vacuum, then (h^(2)in(0))/(me^(2)) has the unit

SOLVED: The quantum-mechanical treatment of the Hatom gives the energy; E; of the electron as 3 function of n: E = 87 - ?mea3n? (n =1,2,3,..) where h is Plancks constant; me
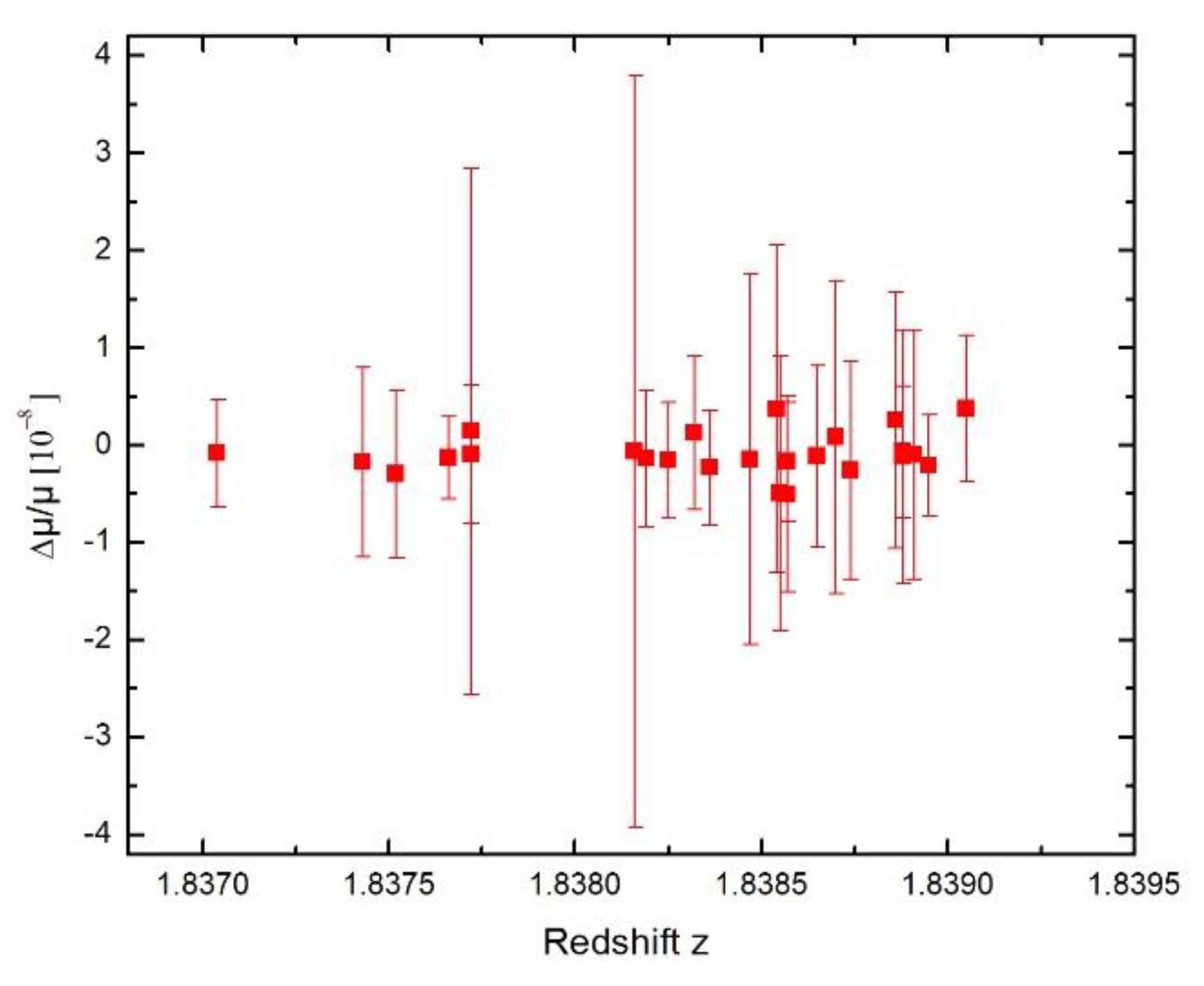
Symmetry | Free Full-Text | New Limit on Space-Time Variations in the Proton-to-Electron Mass Ratio from Analysis of Quasar J110325-264515 Spectra
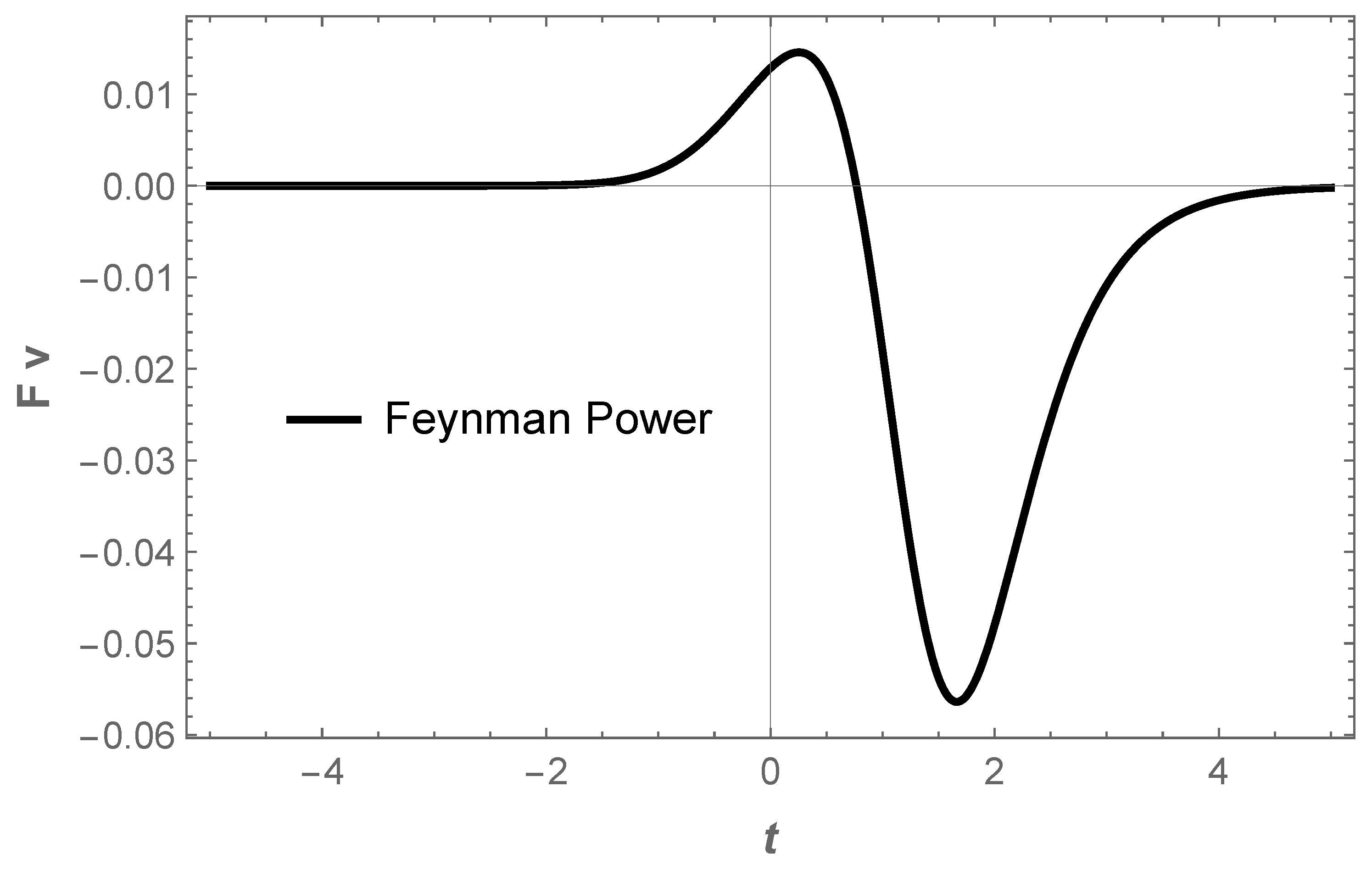
Physics | Free Full-Text | Electron as a Tiny Mirror: Radiation from a Worldline with Asymptotic Inertia

For a two dimensional particle in a box and we have this molecule, with 26 pi electrons, what should the n's be in the Schrodinger equation? In other words, which orbitals are
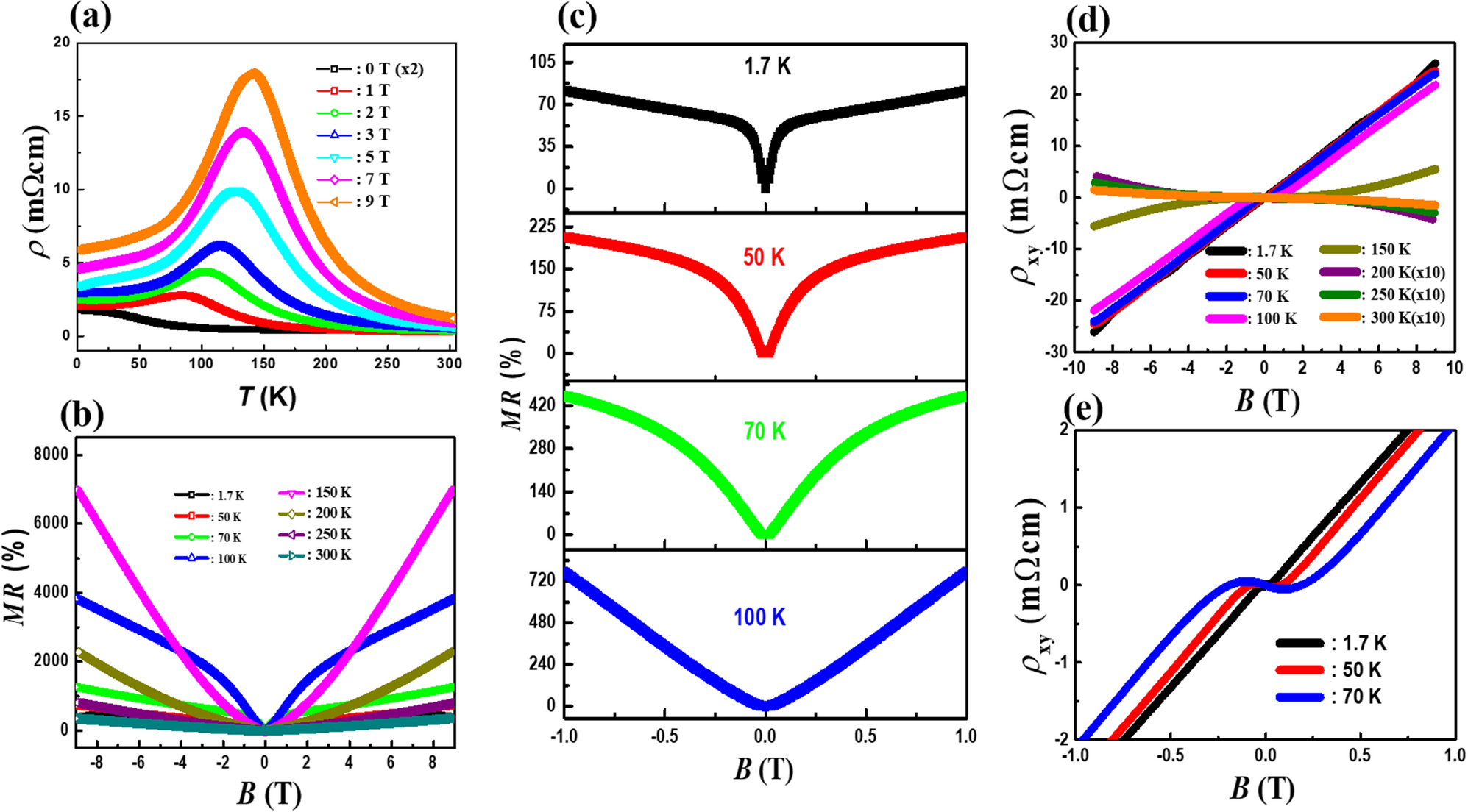
Weak antilocalization, spin–orbit interaction, and phase coherence length of a Dirac semimetal Bi0.97Sb0.03 | Scientific Reports

PDF) Planck Calculation of both Proton and Electron Mass Understanding Relationship as (re/a0)3/4, also (α2)3/4, 'Mass' Scaling Method of 1,602:1 further Applied with the Pauli-Hemisphere Pair Physical Model Generating Settling at 8/7+Anamolous