Table 4 from Nickel(II) complexes with tetra- and pentadentate aminopyridine ligands: synthesis, structure, electrochemistry, and reduction to nickel(I) species. | Semantic Scholar

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa
The standard oxidation potential of Ni|Ni2+ electrode = 0.236 V. If this is combined with a hydrogen electrode in acid solution, at what pH of the solution will the measured emf be
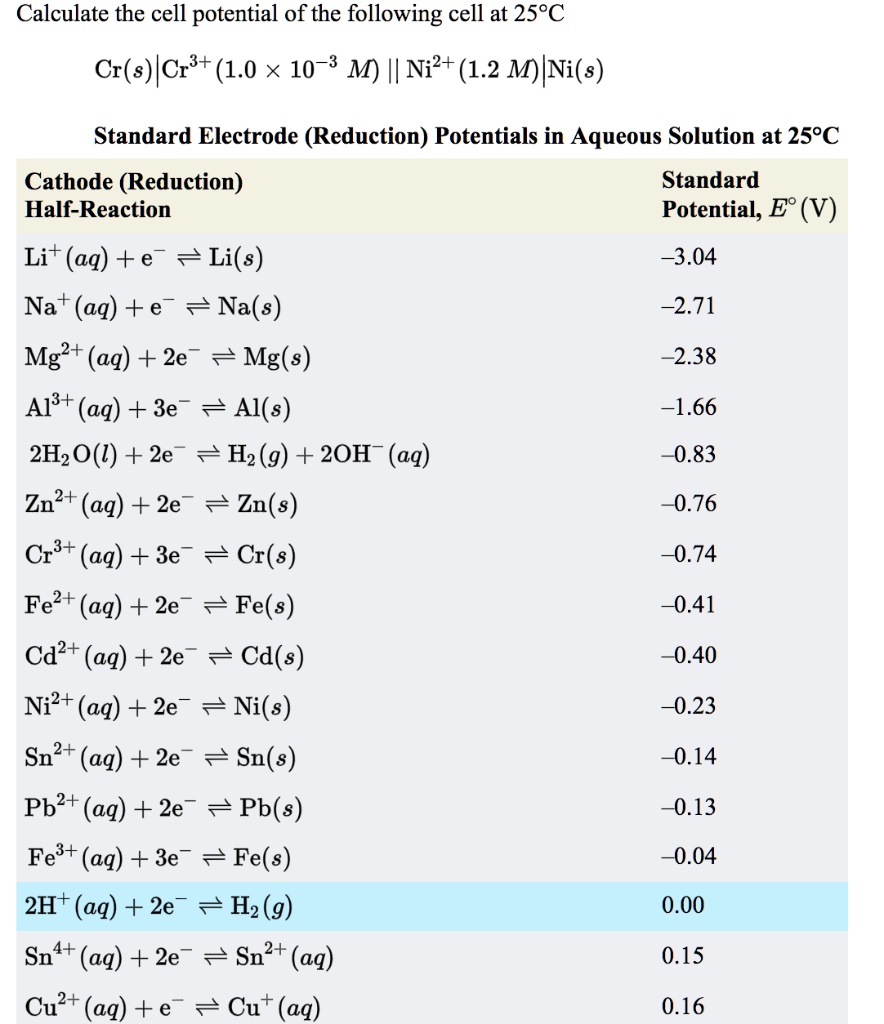
SOLVED: Calculate the cell potential of the following cell at 258C Cr(s)ICr8+(1.0 10-: M) || Ni2+ (1.2 M) Ni(s) Standard Electrode (Reduction) Potentials in Aqueous Solution at 259€ Cathode (Reduction) Standard Half-Reaction

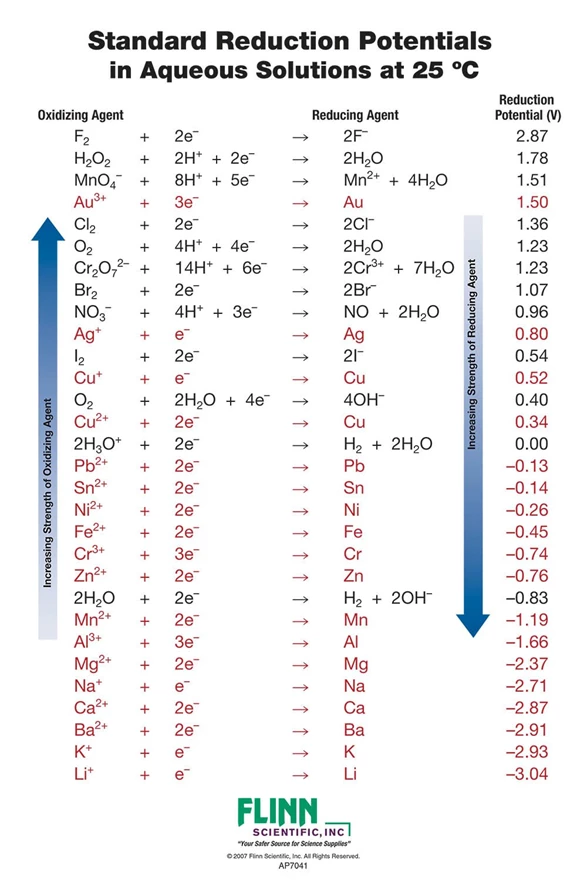
Standard reduction potential of different species in aqueous solution... | Download Scientific Diagram

Standard Reduction Potential (E): when given two half reactions and then asked to give E for a reac… | Reduction potential, Chemistry education, Across the universe

The standard electrode potentials of Zn and Ni respectively are - 0.76 V and - 0.25 V. Then the standard emf of the spontaneous cell by coupling these under standard conditions is:

Calculate the standard electrode potential of Ni2+/Ni electrode if |Class 12 CHEMISTRY | Doubtnut - YouTube